Sponsored: One expert weighs in on an innovative CIDP treatment
By argenx · 3 min read
Living with chronic inflammatory demyelinating polyneuropathy (CIDP) can be difficult. From finding the right treatment to setting the right treatment goals and everything in between, there can be a lot to consider.
Dr. Niraja Suresh, a board-certified neurologist from Tampa, Florida, talked with us about her experience treating people living with CIDP, her approach to having meaningful conversations, and her involvement in the clinical study for VYVGART Hytrulo—the first major innovation in CIDP treatment in over 30 years.*
*VYVGART Hytrulo contains efgartigimod alfa and hyaluronidase and is specifically designed to attach to FcRn receptors.
FcRn=neonatal Fc receptor
A one-of-a-kind treatment for a one-of-a-kind you
Dr. Suresh’s involvement in the VYVGART Hytrulo clinical study gave her a unique perspective on the innovative treatment. It’s the first and only plasma-free treatment approved by the FDA for CIDP. The study was designed to establish the safety and effectiveness of VYVGART Hytrulo for adults with CIDP. It was the largest clinical study in CIDP history and included people with different types of CIDP.
The VYVGART Hytrulo study was conducted in 2 stages: A and B. Stage A aimed to identify patients who showed symptom improvements with VYVGART Hytrulo at 2 consecutive visits. Stage B’s goal, the main goal of the clinical study, was to determine how long arm and leg function was maintained without getting worse for patients taking VYVGART Hytrulo, compared to those who received placebo.
Here's what the study found:
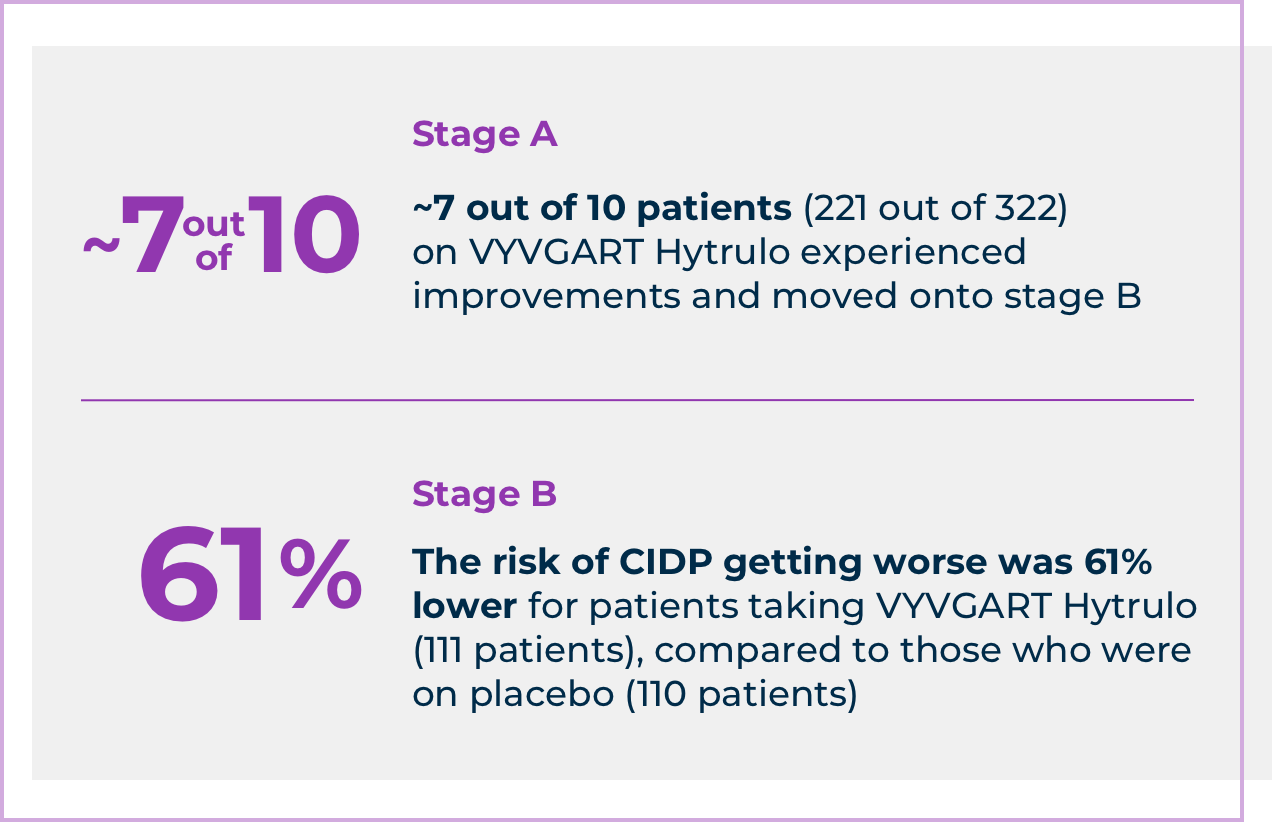
“The patients who continued on VYVGART Hytrulo maintained their level of arm and leg function longer than those who received placebo. And with a disease like CIDP, this is a huge piece of good news, in my opinion,” said Dr. Suresh.
Select safety information of VYVGART Hytrulo
The most common side effects of VYVGART Hytrulo are respiratory tract infection, headache, urinary tract infection, tingling sensation, muscle pain, and injection site reaction. These are not all the possible side effects of VYVGART Hytrulo. You should talk to your doctor about all possible side effects before making any treatment decisions. Please see additional important safety information at the end of this article.

Download your easy-to-use CIDP Doctor Discussion Guide
The first subcutaneous injection approved for CIDP
VYVGART Hytrulo is one weekly injection given under the skin, that takes about 30 to 90 seconds to inject by a healthcare professional.† The injection doesn’t require vein access, and in some cases, may be given at home by a trained nurse.
†Your doctor will monitor you for allergic reactions for 30 minutes after the injection.
So, how do you talk to your doctor about VYVGART Hytrulo and your CIDP treatment goals?
Dr. Suresh knows it can be hard to talk to your doctor. Below are a few examples of what helped her better understand her patients with CIDP.

Sharing your experience with CIDP can be deeply personal. But when you’re open with your care team, they can help you create a treatment routine.
IMPORTANT SAFETY INFORMATION
Do not use VYVGART HYTRULO if you have a serious allergy to efgartigimod alfa, hyaluronidase, or any of the other ingredients in VYVGART HYTRULO. VYVGART HYTRULO can cause serious allergic reactions and a decrease in blood pressure leading to fainting.
VYVGART HYTRULO may cause serious side effects, including:
- Infection. VYVGART HYTRULO may increase the risk of infection. The most common infections for efgartigimod alfa-fcab-treated patients were urinary tract and respiratory tract infections. Signs or symptoms of an infection may include fever, chills, frequent and/or painful urination, cough, pain and blockage of nasal passages/sinus, wheezing, shortness of breath, fatigue, sore throat, excess phlegm, nasal discharge, back pain, and/or chest pain.
- Allergic Reactions (hypersensitivity reactions). VYVGART HYTRULO can cause allergic reactions such as rashes, swelling under the skin, and shortness of breath. Hives were also observed in patients treated with VYVGART HYTRULO. Serious allergic reactions, such as trouble breathing and decrease in blood pressure leading to fainting have been reported with efgartigimod alfa-fcab.
- Infusion-Related Reactions. VYVGART HYTRULO can cause infusion-related reactions. The most frequent symptoms and signs reported with efgartigimod alfa-fcab were high blood pressure, chills, shivering, and chest, abdominal, and back pain.
Tell your doctor if you have signs or symptoms of an infection, allergic reaction, or infusion-related reaction. These can happen while you are receiving your VYVGART HYTRULO treatment or afterward. Your doctor may need to pause or stop your treatment. Contact your doctor immediately if you have signs or symptoms of a serious allergic reaction.
Before taking VYVGART HYTRULO, tell your doctor if you:
- take any medicines, including prescription and non-prescription medicines, supplements, or herbal medicines,
- have received or are scheduled to receive a vaccine (immunization), or
- have any allergies or medical conditions, including if you are pregnant or planning to become pregnant, or are breastfeeding.
What are the common side effects of VYVGART HYTRULO?
The most common side effects in efgartigimod-alfa-fcab-treated patients were respiratory tract infection, headache, and urinary tract infection. Additional common side effects with VYVGART HYTRULO are injection site reactions, including rash, redness of the skin, itching sensation, bruising, pain, and hives.
These are not all the possible side effects of VYVGART HYTRULO. Call your doctor for medical advice about side effects. You may report side effects to the US Food and Drug Administration at 1-800-FDA-1088.
What is VYVGART® HYTRULO (efgartigimod alfa and hyaluronidase-qvfc)?
VYVGART HYTRULO is a prescription medicine used for the treatment of adult patients with chronic inflammatory demyelinating polyneuropathy (CIDP)
Please see the full Prescribing Information for VYVGART HYTRULO and talk to your doctor.
Visit VYVGARTHytruloCIDP.com/glossary for a glossary of terms.
A note from RareDisease.net: The content of this article was provided by our sponsor. RareDisease.net does not specifically endorse or recommend the program, product, medications, or therapies discussed in this article.
VYVGART is a registered trademark of argenx.
VYVGART Hytrulo is a trademark of argenx.
For U.S. audiences only.
©2024 argenx US-VYV_HYT-24-00260 V1 11/2024

